When your doctor prescribes a generic drug, you expect it to work just like the brand-name version. After all, the FDA says it’s the same. But what if it doesn’t? What if the pill you’re taking every morning isn’t doing what it’s supposed to-and your condition is getting worse?
Why a Generic Might Not Work
Generic drugs are required to contain the same active ingredient as the brand-name version. That’s the law. But here’s the catch: the law doesn’t require them to work the same way. The FDA allows generics to differ in how quickly or completely they release that active ingredient into your body, as long as it’s within an 80% to 125% range of the original. That’s a 45% window. For most medications, that’s fine. But for drugs with a narrow therapeutic index-like warfarin, phenytoin, digoxin, or tacrolimus-that margin is dangerous.Take warfarin, for example. A 5% change in blood levels can mean the difference between a clot forming in your leg or bleeding uncontrollably inside your brain. If a generic version releases the drug too fast, you’re at risk of bleeding. Too slow, and you’re unprotected. Patients who’ve switched from brand to generic have reported sudden clots, strokes, or hemorrhages-all because the drug didn’t behave the same way in their bodies.
The Hidden Problem: Inactive Ingredients
You might think the only thing that matters is the active ingredient. But it’s not that simple. The fillers, binders, and coatings-called inactive ingredients-can change how a pill breaks down in your stomach. One study found that some generic versions of the ADHD drug Concerta dissolved over three times faster than the brand. That means the medication hits your bloodstream all at once instead of slowly over the day. The result? A spike in side effects, followed by a sudden drop in effectiveness.In 2013, the FDA pulled Budeprion XL, a generic version of Wellbutrin, after hundreds of patients reported severe mood swings, anxiety, and depression. The problem? The inactive ingredients changed how the drug was released. The same active ingredient-bupropion-was there. But the pill didn’t work the same. And patients paid the price.
Manufacturing Chaos
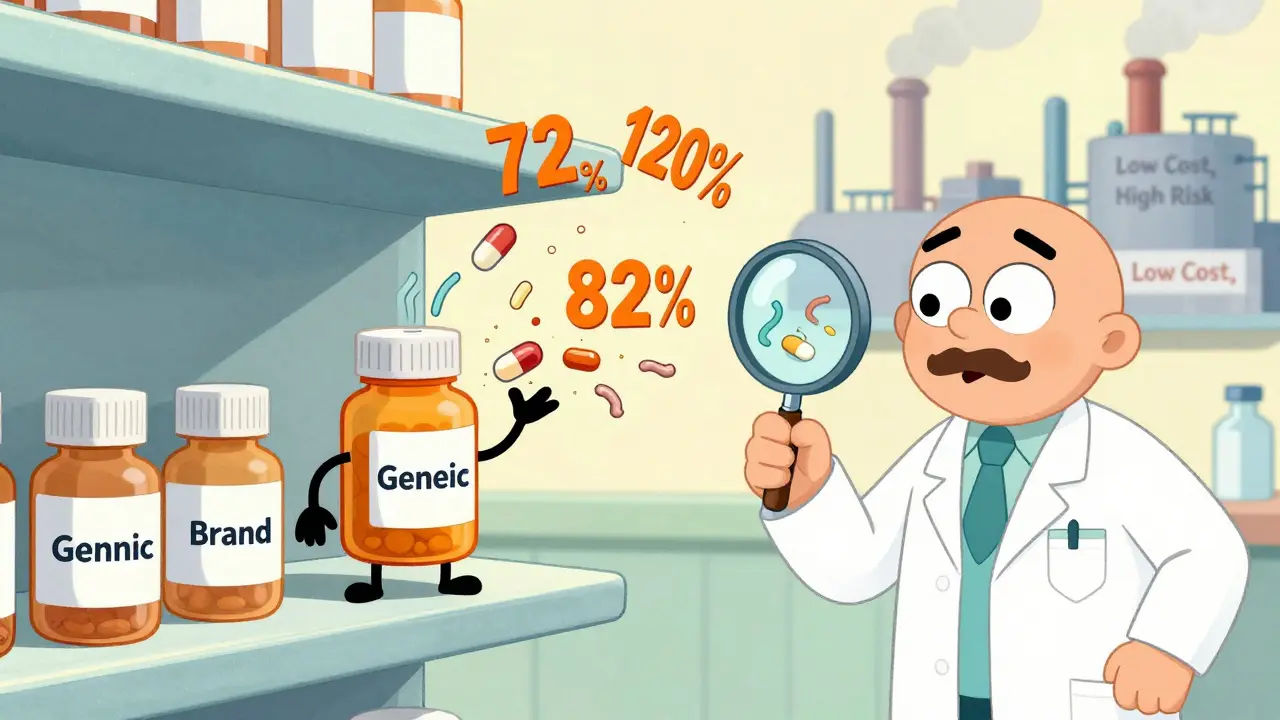
Most generic drugs are made overseas, often in countries with weaker oversight. A 2025 investigation by STAT News found that some chemotherapy generics contained as little as 72% of the labeled dose. Others had over 112%. In one case, pills from the same blister pack had wildly different amounts of active ingredient. That’s not a mistake. That’s a systemic failure.Manufacturing defects account for nearly a third of all problems flagged in generic drug applications. Some pills don’t dissolve properly. Others degrade in heat or humidity. Potassium chloride tablets from Glenmark Pharmaceuticals were recalled in 2024 because they weren’t breaking down in the gut-leaving patients with dangerous electrolyte imbalances. If the drug doesn’t dissolve, it doesn’t get absorbed. And if it doesn’t get absorbed, it doesn’t work.
NTI Drugs: Where the System Breaks Down
Drugs with a narrow therapeutic index are the most dangerous when generics go wrong. These are medications where the dose that helps is almost the same as the dose that kills. For these, the FDA should require tighter standards-like a 90% to 111% bioequivalence range. But they don’t always enforce it.A 2024 study of multiple sclerosis patients showed a clear pattern. Those whose disease stayed stable were taking generics with 97% to 103% of the correct dose. Those who relapsed? Their pills had 91%, 82%, and even 73% of the needed drug. One patient ended up in the hospital with a full-blown flare-up. Her doctor didn’t know why-until he checked the batch number on the bottle.
Real Stories, Real Consequences
A heart transplant patient in Brisbane switched to a generic tacrolimus after her insurance denied coverage. Within weeks, she felt exhausted, short of breath, and nauseous. Her transplant team ran tests. Her drug levels were too low. She was at risk of rejecting her new heart. She had to go back to the brand-name version-costing her $600 a month instead of $30. But she was alive.A cancer patient in Queensland was given a generic version of methotrexate. After the first dose, she couldn’t keep anything down. Her white blood cell count crashed. Her oncologist assumed it was a side effect. It wasn’t. The generic contained 120% of the labeled dose. She was overdosing. She had to stop treatment for six weeks while her body recovered. By then, the cancer had grown.
Pharmacists are seeing this more often. They report patients who say, “This isn’t the same.” They’ve had people come in with rashes, seizures, or sudden organ failure after switching to a new generic batch. Sometimes, the only way to fix it is to switch back-even if it means paying more.

Who’s Responsible?
The blame doesn’t lie with one person. It’s a system. Pharmacy Benefit Managers (PBMs) push for the cheapest generics, often without knowing if they’re safe. Manufacturers cut corners to keep prices low. Regulators rely on outdated testing methods. And patients? They’re left guessing why their treatment isn’t working.There’s no central database tracking which generic batches cause problems. No warning labels. No mandatory reporting from doctors or pharmacists. If a patient has a bad reaction, it’s chalked up to “disease progression.” But in too many cases, it’s not the disease. It’s the drug.
What You Can Do
If you’re on a generic drug and something feels off-worse side effects, less effectiveness, new symptoms-don’t assume it’s just you. Talk to your doctor. Ask:- Is this drug on the list of those with a narrow therapeutic index?
- Has the manufacturer changed recently?
- Can we try the brand-name version-even temporarily-to see if things improve?
Keep a journal. Note when you started the generic, what symptoms you had before, and what changed after. Bring it to your appointment. That data can save your life.
Some insurers will cover the brand-name version if you prove the generic isn’t working. You have to ask. And you have to push.
The Bigger Picture
The generic drug system was built to save money. And for most people, it works. But for those on critical medications, it’s a gamble. The same companies that make generics also make brand-name drugs. They know the difference. The regulators know the risks. But the system still moves forward-because profit is easier to measure than patient outcomes.Until we demand better testing, stricter standards, and full transparency in the supply chain, people will keep getting sick because a pill didn’t dissolve right. Or because the dose was wrong. Or because the drug degraded in a warehouse halfway across the world.
You deserve a medication that works. Not one that’s just cheap enough.

 Health and Wellness
Health and Wellness
Gaurav Meena
January 29, 2026 AT 18:39Beth Beltway
January 31, 2026 AT 03:34Natasha Plebani
February 1, 2026 AT 14:11Eliana Botelho
February 1, 2026 AT 15:33Rob Webber
February 2, 2026 AT 12:46calanha nevin
February 3, 2026 AT 08:04Lisa McCluskey
February 3, 2026 AT 23:27owori patrick
February 4, 2026 AT 02:00Darren Gormley
February 5, 2026 AT 19:02