
Long COVID Treatment Effectiveness Calculator
Your Symptoms
Select symptoms that apply to you (select all that apply)
Your Health Conditions
Select any pre-existing conditions that apply
Treatment Options
Select treatments to compare (select multiple)
Treatment Analysis
More than 15 million people in the U.S. alone are living with Long COVID - symptoms that won’t go away weeks or months after the initial infection. Fatigue, brain fog, heart palpitations, shortness of breath, and muscle pain aren’t just lingering side effects. For many, they’re life-altering. And right now, there’s no FDA-approved treatment for it. That’s why doctors, researchers, and patients are turning to existing medications - repurposing drugs meant for other conditions - in hopes of finding relief. But here’s the catch: we don’t fully know what these drugs do to people with Long COVID. Safety signals are emerging, and some are worrying.
What’s Actually Being Tried Right Now?
The biggest research effort in the world for Long COVID is the NIH’s RECOVER initiative. It’s spent over $1 billion since 2021, funding dozens of trials. Among the most studied drugs are baricitinib, metformin, low-dose naltrexone (LDN), and Paxlovid - all repurposed from other uses.
Baricitinib, a JAK inhibitor approved for rheumatoid arthritis and alopecia, is being tested in a phase 3 trial called REVERSE-LC. It worked in severe acute COVID by calming an overactive immune system. Early data suggested it might help with Long COVID fatigue and brain fog. But here’s what we know about safety: in arthritis patients, it raised the risk of serious infections by 10-20%, increased heart attacks and strokes, and showed a small but real link to lymphoma. Long COVID patients are often younger and healthier than rheumatoid arthritis patients. So does that mean baricitinib is safer for them? Maybe. But we don’t have the data yet. Results from this trial won’t be out until late 2026.
Metformin, a cheap, widely used diabetes drug, has the strongest evidence so far. A 2023 University of Minnesota trial found it cut the risk of developing Long COVID by 41% when taken within the first week of infection. That’s huge. But the side effects? Rough. Over a third of people had nausea, diarrhea, or stomach pain. For someone already dealing with fatigue and digestive issues from Long COVID, that’s a tough trade-off. It’s not a cure, but it’s one of the few drugs with real clinical trial backing.
Low-dose naltrexone (LDN) is being used off-label by many patients. Typically, naltrexone is given at 50 mg for opioid addiction. LDN uses 1-5 mg daily - a tiny fraction. In a 2024 patient survey from Nova Southeastern University, 62% reported less fatigue. But 28% had trouble sleeping, and 19% got headaches. These aren’t lab results - they’re real people reporting how they feel. That’s valuable, but it’s not proof. No large, controlled trial has confirmed these benefits yet.
Paxlovid, the antiviral combo of nirmatrelvir and ritonavir, was a game-changer for acute COVID. But for Long COVID? The data is mixed. One small UCSF study found symptom improvement in 38% of people taking a 15-day course. But a much larger NIH trial found no difference compared to placebo. And the side effects? A bitter taste affected nearly 80% of users. Ritonavir also interferes with dozens of common medications - statins, blood thinners, even some anxiety drugs. For someone already on multiple prescriptions, that’s a red flag.
What’s Failing - and Why It Matters
Not every drug being tested works. In March 2025, the BC007 trial was stopped. It was designed to neutralize autoantibodies thought to drive Long COVID symptoms. But it didn’t beat placebo. Worse, three people in the treatment group had serious infusion reactions - compared to just one in the placebo group. That’s a red flag. When a drug looks promising in theory but fails in practice, it tells us something: Long COVID isn’t one disease. It’s many.
Another drug, AER002, a human immunoglobulin, is still in phase 2. So far, mild infusion reactions happened in 18% of patients. No serious events. That’s promising. But we’re still in early stages. And then there’s polymerized type I collagen, tested in a small Johns Hopkins pilot. Only 12% reported injection site pain. No serious side effects. It’s a novel approach - trying to repair damaged tissue. But it’s only been tested in 40 people. Too early to call it a breakthrough.

The Big Unknowns
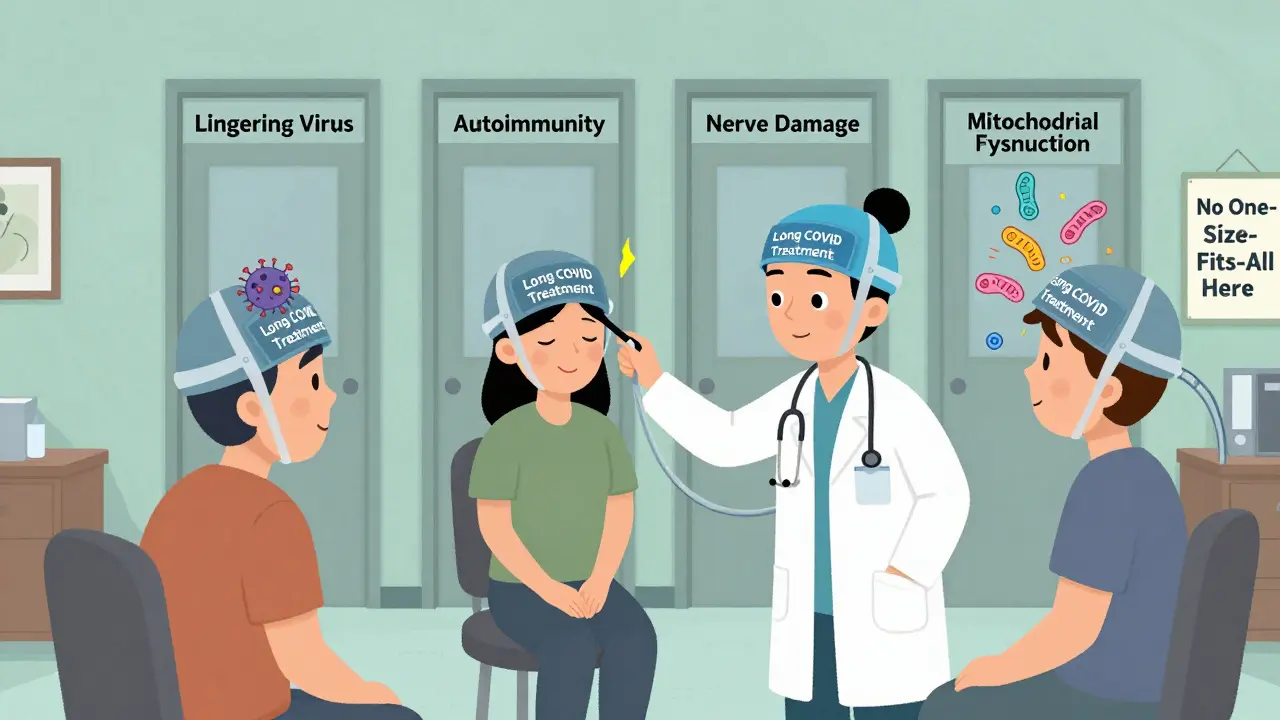
We don’t know who will respond to what. That’s the biggest problem. Long COVID isn’t a single condition. The NIH now recognizes at least four different types - some driven by lingering virus, others by autoimmunity, nerve damage, or mitochondrial dysfunction. One drug might help one group and hurt another.
There’s also no lab test to confirm Long COVID. No blood marker. No scan. No clear way to tell if a treatment is working. Doctors are guessing. Patients are guessing. That makes clinical trials harder. If you can’t measure improvement, how do you prove a drug works?
And then there’s the safety gap. Most of these drugs were tested in older, sicker populations - people with rheumatoid arthritis, diabetes, or cancer. Long COVID patients are often younger, previously healthy, and not on other immunosuppressants. Does baricitinib’s infection risk apply the same way? Maybe not. But we don’t know. We’re using drugs designed for different people, in different bodies, for different reasons.
What About New Drugs?
Some researchers are looking beyond repurposing. The WEHI Institute in Australia found a new antiviral compound in mice that prevented Long COVID symptoms entirely. But it’s not tested in humans yet. Toxicity studies start in early 2026. That’s years away from being available.
Other experimental ideas include GLP-1 receptor agonists like tirzepatide (Mounjaro), approved for diabetes and weight loss. These drugs reduce inflammation and may help with brain fog and fatigue. But they cause nausea, vomiting, and diarrhea - side effects that could make Long COVID worse, not better. Trials are just beginning.
Then there’s the stellate ganglion block - a nerve injection used for chronic pain. Some patients report relief from anxiety, heart palpitations, and sweating. But the procedure carries risks: hoarseness in 15% of cases, bleeding in 5%. No large study has looked at safety in Long COVID patients. It’s being tested now, but it’s still experimental.
What Are Patients Actually Doing?
While trials are ongoing, people aren’t waiting. The Body Politic Long COVID Support Group - with over 15,000 members - surveyed its community in 2025. Sixty-eight percent have tried at least one medication off-label. The top three? Metformin (32%), LDN (29%), and Paxlovid (24%).
But here’s the sobering part: 57% said they didn’t get enough relief. And 41% said the side effects were worse than their symptoms. For metformin, stomach issues were the main complaint. For LDN, sleep problems. For Paxlovid, the bitter taste and drug interactions made it hard to keep taking.
One patient in Brisbane told me: “I took metformin for three weeks. I lost five pounds. My brain fog didn’t change. But I couldn’t eat without feeling sick. I stopped.”
Another said: “LDN gave me back energy. But I couldn’t sleep. I felt like I was on a rollercoaster. I’m still trying to figure out if it’s worth it.”
What Should You Do?
If you have Long COVID and are considering medication, here’s what you need to know:
- Don’t self-prescribe. Even “safe” drugs like LDN or metformin can interact with other meds or worsen symptoms.
- Track your symptoms. Use a journal or app. Note when you start a drug, what you feel, and how you sleep, eat, and think. This helps your doctor spot patterns.
- Ask about clinical trials. RECOVER has sites across the U.S. and Australia. You might get access to drugs before they’re widely available - and you’ll be monitored closely.
- Be patient with uncertainty. We’re learning as we go. What works for one person may not work for you. That doesn’t mean you’re failing. It means the science is still evolving.
The FDA won’t approve a Long COVID treatment until we have clear evidence of benefit - and a solid understanding of risk. That’s coming. But not yet. In the meantime, the best thing you can do is stay informed, work with a doctor who understands Long COVID, and avoid treatments that promise miracles.
The path forward isn’t about one magic pill. It’s about matching the right treatment to the right person - and knowing when not to use a drug, even if it seems like it should help.
Is there any FDA-approved medication for Long COVID yet?
No, there are currently no FDA-approved medications specifically for Long COVID. All current treatments are used off-label or are still in clinical trials. The NIH’s RECOVER initiative is leading the largest effort to find effective therapies, with results from major trials expected in 2026 and 2027.
Can I take metformin to prevent Long COVID after having COVID?
The University of Minnesota’s STOP COVID trial showed metformin reduced the chance of developing Long COVID by 41% when taken within the first week of infection. But it’s not yet a standard recommendation. Talk to your doctor before starting it - especially if you have kidney issues, are pregnant, or have a history of stomach problems. Side effects like nausea and diarrhea are common and can be hard to manage.
Is low-dose naltrexone (LDN) safe for Long COVID?
LDN is used off-label and has shown symptom improvement in some patients, particularly for fatigue and pain. But it’s not FDA-approved for Long COVID. Reported side effects include sleep disturbances (28%) and headaches (19%). There’s no long-term safety data in this population. Use only under medical supervision, and avoid it if you’re taking opioids or have liver disease.
Why do some doctors recommend baricitinib while others warn against it?
Baricitinib reduces inflammation and worked in severe acute COVID, so some see it as a logical choice for Long COVID. But it carries risks: serious infections, blood clots, and possible cancer links. Doctors who support it believe the benefits outweigh risks in carefully selected patients. Others warn that Long COVID patients may already have immune dysregulation, making them more vulnerable to these side effects. Until phase 3 trial results are published in late 2026, the decision remains highly individualized.
Should I try Paxlovid for Long COVID if it worked for my acute infection?
Paxlovid was designed to stop viral replication early. In Long COVID, the virus is likely no longer active - so the drug may not help. Two major studies gave conflicting results. One showed slight benefit; the other showed none. Plus, the bitter taste and drug interactions make it hard to tolerate long-term. It’s not recommended as a standard Long COVID treatment, but some doctors may try it in specific cases. Always check for medication interactions with your pharmacist.

 Health and Wellness
Health and Wellness
Ashley S
January 5, 2026 AT 14:51This is why I don't trust doctors anymore. They just throw pills at us like we're lab rats. I took metformin for two weeks and felt like I was gonna puke all day. No brain fog fix. Just a bloated, miserable mess. Why are we even trying this?
Rachel Wermager
January 5, 2026 AT 19:20The pathophysiological mechanisms underlying Long COVID are heterogenous, necessitating stratified therapeutic approaches. Baricitinib’s JAK-STAT modulation may attenuate cytokine-driven neuroinflammation in the CNS subset, yet its thrombotic risk profile in young, non-immunocompromised cohorts remains inadequately characterized. The REVERSE-LC trial’s primary endpoint-fatigue reduction via PFS-3-is statistically underpowered for subgroup analysis. We need biomarker-guided enrollment, not population-wide off-label prescribing.
Katelyn Slack
January 6, 2026 AT 00:36i just wanted to say thank you for writing this. i’ve been dealing with this for 2 years and no one ever talks about how scary it is to try stuff that might make you worse. i tried ldn and it gave me energy but i couldnt sleep for weeks. i stopped. i feel seen. thanks.
Melanie Clark
January 6, 2026 AT 08:53They’re hiding the truth. The CDC knows this is all from the spike protein lingering in the gut and the mRNA is still in your cells. They don’t want you to know because Big Pharma profits off the chaos. LDN works because it resets your immune system but they banned it in 3 states because it’s too cheap. Paxlovid? That’s just a placebo with a bitter taste to keep you buying. I’ve been tracking my symptoms since 2021. The numbers don’t lie. The system is rigged. Wake up.
Harshit Kansal
January 8, 2026 AT 06:44Bro i took metformin for 10 days just to see what happens. My stomach was screaming. I lost 3kg. My brain fog stayed the same. I stopped. I think the real fix is rest and time. Not more pills. We need to stop treating this like a math problem.
Brian Anaz
January 8, 2026 AT 21:55Why are we wasting billions on this? In my day, you just dealt with it. People got sick, they recovered, or they didn’t. Now we have a whole industry built around people who won’t just move on. This is weakness. We should be focusing on real problems. Not giving pills to people who can’t handle a little fatigue.
Matt Beck
January 10, 2026 AT 14:07It’s not about the drugs… it’s about the *meaning* we assign to suffering. We live in a world that equates healing with chemical intervention… but what if the body is trying to *teach* us something? What if Long COVID is the soul’s rebellion against modernity? LDN didn’t fix my fatigue… it made me stop running. Maybe the real cure is stillness… not a pill… 🌿✨
Molly McLane
January 11, 2026 AT 20:22Hey everyone - I’ve been in the Long COVID community for 3 years. I’ve tried metformin, LDN, even the stellate ganglion block. None of it fixed everything. But here’s what helped: finding a doctor who listens. Keeping a symptom journal. Saying no to pressure to 'just push through'. You’re not broken. You’re not lazy. You’re not failing. We’re all just trying to figure this out together. One day at a time.
Tiffany Adjei - Opong
January 12, 2026 AT 08:16Actually, the NIH trial on Paxlovid was flawed because they didn’t account for viral persistence in tissue reservoirs. And LDN? It’s not 'off-label' - it’s a well-documented immunomodulator used in MS and fibromyalgia for decades. The real issue is that pharma doesn’t own the patent, so nobody funds big trials. That’s why you only hear about metformin - it’s cheap and generic. Wake up - this is about money, not medicine.
Ryan Barr
January 13, 2026 AT 02:27Metformin works. LDN doesn’t. Baricitinib is dangerous. Paxlovid is useless. End of story.