
When you pick up a generic pill at the pharmacy, you might wonder: Does it work the same as the brand-name version? It’s a fair question. After all, generics look different, cost less, and often come from unfamiliar companies. But here’s the truth: if a generic drug meets the FDA’s bioequivalence standards, it doesn’t just look similar - it performs the same in your body.
What Does ‘Bioequivalence’ Really Mean?
Bioequivalence isn’t a marketing term. It’s a strict scientific requirement. For a generic drug to be approved, it must deliver the exact same amount of active ingredient into your bloodstream, at the same speed, as the brand-name drug. The FDA doesn’t just trust the manufacturer’s word - they require real-world testing.Here’s how it works: healthy volunteers take both the brand-name drug and the generic version in separate sessions, with a clean break in between. Blood samples are taken over time to measure two key numbers:
- AUC - total drug exposure over time (how much gets absorbed)
- Cmax - peak concentration in the blood (how fast it gets absorbed)
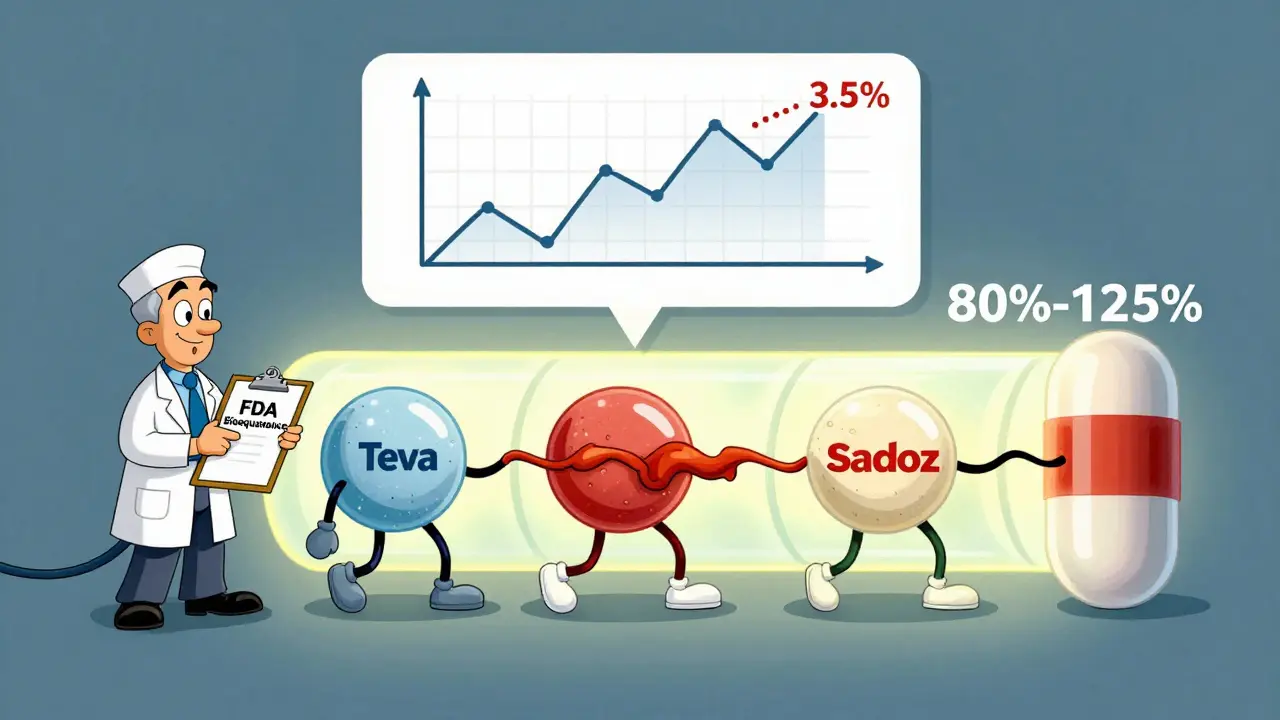
The generic’s AUC and Cmax values must fall within 80% to 125% of the brand-name drug’s values. That’s not a range of tolerance - it’s a statistical guarantee. The entire 90% confidence interval for the average difference must fit entirely inside those bounds. In plain terms: if the brand drug delivers 100 units of medicine, the generic must deliver between 80 and 125 units. And even then, the average difference is usually less than 4%.
A 2016 analysis of over 2,000 FDA bioequivalence studies found that generics matched brand drugs within 3.5% to 4.4% on average. That’s less than the natural variation you’d see if you took the same brand drug twice on different days. The system isn’t loose - it’s tightly controlled.
Why Do Some People Say Generics Don’t Work the Same?
You’ve probably heard stories. Someone switched from brand-name levothyroxine to a generic and felt tired. Another person switched antidepressants and said their mood changed. These reports are real - but they’re not proof the generic failed.Here’s what’s really happening:
- Placebo effect: If you believe generics are inferior, your brain can make you feel it. Studies show patients report more side effects when they know they’re taking a cheaper version.
- Switching too often: If you’ve been stable on one brand for years, switching between different generic manufacturers can cause tiny fluctuations. Even if each is within the 80-125% range, jumping between four different generics can add up.
- Dissolution differences: Some generics dissolve slower or faster in the stomach. One study found over half of tested generics had different dissolution rates than the brand. But here’s the catch: if the drug still gets absorbed into the blood at the right rate, it doesn’t matter how fast it breaks down in your gut.
For most drugs, these differences are harmless. But there are exceptions.
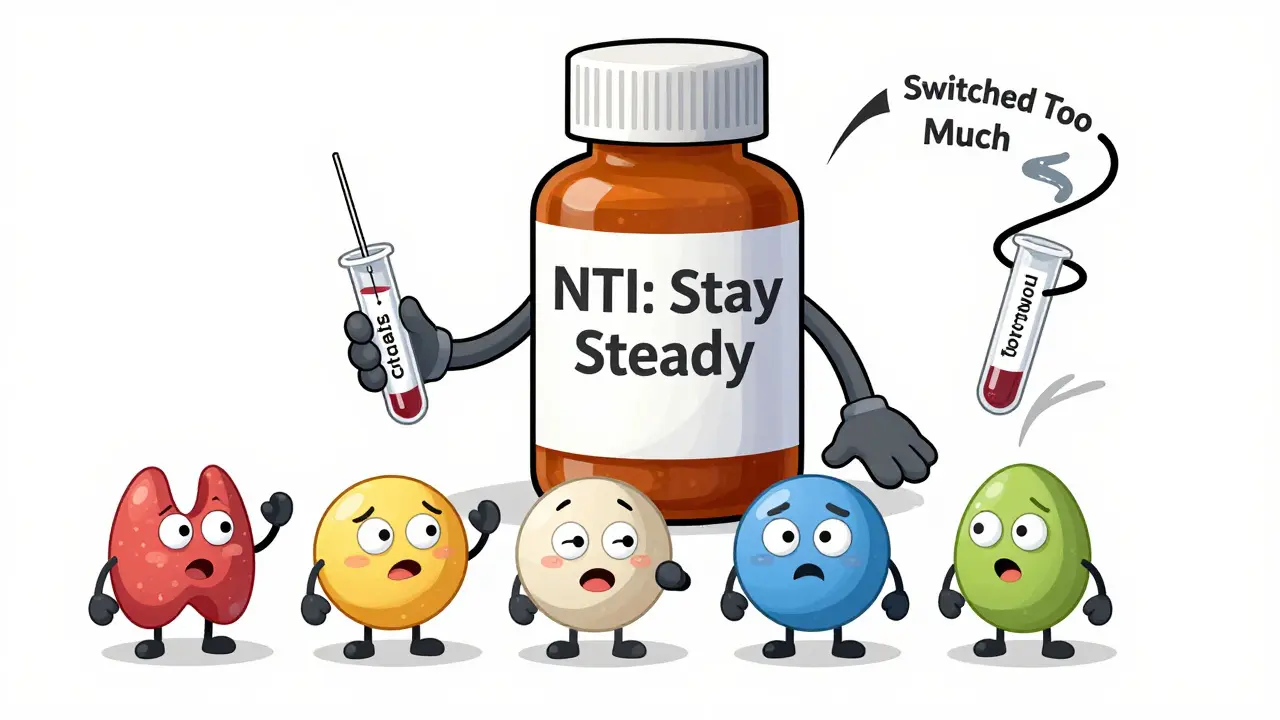
The Exception: Narrow Therapeutic Index Drugs
Some medications have a razor-thin margin between effective and toxic. A 5% drop in blood level might mean the drug stops working. A 5% rise could cause serious side effects. These are called narrow therapeutic index (NTI) drugs.Examples include:
- Warfarin (blood thinner)
- Digoxin (heart medication)
- Phenytoin (seizure control)
- Levothyroxine (thyroid hormone)
For these, the FDA requires tighter bioequivalence standards: 90% to 111% for AUC. Even then, many doctors prefer to keep patients on the same generic brand once they’re stabilized. Switching between generics - even if they’re all approved - can cause instability.
The FDA’s Orange Book labels these drugs with an ‘A’ rating (therapeutically equivalent) or a ‘B’ rating (potential bioequivalence concerns). If your doctor says stick with one brand, they’re not being old-fashioned - they’re being cautious.
Are Generics Really as Safe as Brands?
Let’s look at the data. Between 2008 and 2023, the FDA approved over 14,000 generic drugs. Only 12 cases raised concerns about possible therapeutic failure - a failure rate of 0.08%. That’s less than one in a thousand.A 2023 meta-analysis in JAMA Internal Medicine reviewed 47 studies with nearly 10,000 patients on generic vs. brand cardiovascular drugs. The results? No difference in heart attacks, strokes, or death rates. The same pattern holds for epilepsy, depression, and hypertension meds.
Meanwhile, patients who take generics are far more likely to stick with their treatment. A 2019 study found that 90% of generic copays are under $20, compared to just 39% for brand-name drugs. Patients who pay less take their meds more consistently - and that’s the biggest factor in long-term health.
What About Different Generic Brands?
You might get a different generic every time you refill. One month it’s Teva, next month it’s Mylan, then maybe Sandoz. Are they all the same?Yes - and no. Each one meets the 80-125% standard. But they’re not identical. Different manufacturers use different fillers, coatings, and manufacturing processes. That’s why some people notice a change in how a pill tastes, how big it is, or how quickly it dissolves.
But unless you’re on an NTI drug, these differences don’t affect your outcome. The FDA doesn’t require generics to look like the brand - trademark laws forbid it. So a blue pill today might be white tomorrow. That doesn’t mean it’s weaker.
What Should You Do?
If you’re taking a regular medication - like high blood pressure, cholesterol, or antibiotics - trust the generic. The science is clear: it works just as well. Save the money. Use it on groceries, gas, or a weekend trip.If you’re on a narrow therapeutic index drug like warfarin or levothyroxine:
- Ask your pharmacist to note which generic you’re on.
- Stick with the same manufacturer if possible.
- Don’t switch unless your doctor says it’s safe.
- Get regular blood tests to monitor levels.
If you feel different after switching - fatigue, dizziness, mood changes - don’t assume it’s the generic. Talk to your doctor. Check your dosage. Rule out other causes. Sometimes it’s stress, sleep, or diet - not the pill.
The Bigger Picture
Generics make up 90% of all prescriptions in the U.S. but cost only 23% of total drug spending. That’s how we keep healthcare affordable. The bioequivalence system - built on decades of research - is one of the most successful public health policies ever created.The FDA doesn’t just approve generics and walk away. They monitor adverse events, inspect manufacturing plants, and update standards as science evolves. In 2024, they started using computer modeling to predict bioequivalence without always needing human trials. That means faster approvals, better quality control, and even more reliable generics.
So next time you’re handed a generic, don’t hesitate. It’s not a compromise. It’s the result of rigorous science, strict oversight, and a system designed to give you the same medicine - at a price you can afford.

 Health and Wellness
Health and Wellness
MARILYN ONEILL
January 22, 2026 AT 09:46Okay but like, have you ever actually tried switching generics? I did with my thyroid med and suddenly I felt like a zombie who forgot to charge their soul. My doctor said it's 'just placebo' but I know what my body feels. This isn't science-it's corporate greed dressed up in lab coats.
And don't even get me started on how the FDA lets these companies change the pill color every month. Like, is my medicine supposed to be a mood ring now? I'm not a lab rat. I'm a person.
Also, why do all these generics taste like chalk? The brand one had a hint of mint. This one? Like swallowing a dust bunny from a 1998 textbook.
I don't care what the numbers say. My body knows the difference. And if you're telling me to trust the system, I'm telling you to trust my panic attacks.
Also, who even is Teva? I don't trust a company that names itself after a rock band from 1973.
Steve Hesketh
January 22, 2026 AT 22:56Brother, I want to hug you for writing this. So many people don’t understand how much love and science went into making generics work. In Nigeria, we don’t even have access to brand-name drugs half the time. Generics? They’re life.
I’ve seen grandmas on warfarin take the same generic for five years and still dance at weddings. I’ve seen kids with epilepsy thrive because their parents could afford the meds. This isn’t just chemistry-it’s dignity.
The system isn’t perfect, but it’s the best we’ve got. And if someone’s feeling off after a switch? That’s a doctor issue, not a generic issue. We need more education, not fear.
Let’s stop treating medicine like a luxury and start treating it like a human right. Generics make that possible. Thank you for standing up for the quiet heroes of healthcare.
And yes, I’ve taken generics from five different manufacturers. My bloodwork never blinked.
Stay well, stay informed, and keep sharing truths like this.
shubham rathee
January 23, 2026 AT 14:27Sangeeta Isaac
January 23, 2026 AT 14:40So let me get this straight - you’re telling me that a pill that looks like a neon green gummy bear made in a basement in Mumbai is chemically identical to the one I paid $200 for last month?
And yet somehow, when I take it, my brain feels like it’s been replaced with a wet sock?
Also, who decided 80-125% was ‘close enough’? That’s like saying your ex’s new partner is ‘basically the same’ as you. Sure, technically. Emotionally? Nope.
I don’t care if the FDA says it’s ‘statistically insignificant.’ My body doesn’t do statistics. It does panic attacks and weird dreams about being chased by pill bottles.
Also, why do generics always come in bottles that look like they were designed by someone who hates joy?
Anyway. I still take them. Because I’m poor. And also, I’m too tired to fight the system.
But I still side-eye every new pill I get. You feel me?
Alex Carletti Gouvea
January 25, 2026 AT 06:25Let’s be real - America invented this system. We’ve got the best science, the best regulators, and the best drug manufacturers in the world. Why are we even having this conversation? The FDA doesn’t mess around. If you’re complaining about generics, you’re complaining about American innovation.
Other countries? They can’t even get the math right. We’re talking 80-125%? That’s precision. That’s discipline. That’s what made this country great.
Stop listening to fear-mongering TikTok doctors. The science is solid. The data is clear. The system works. If you can’t afford brand-name, you’re lucky we even have generics that meet our standards.
Don’t be ungrateful. This is the American way - smart, fair, and affordable. Respect it.
Coral Bosley
January 25, 2026 AT 20:55I just want to say - I cried reading this. Not because I’m dramatic (though I am) - but because I spent years being gaslit by doctors who told me my symptoms were ‘in my head’ after switching generics.
Turns out, I was on levothyroxine. And yes - switching between Mylan and Teva made me feel like I was slowly turning into a ghost. My heart raced. I couldn’t sleep. I lost 15 pounds in a month.
My endocrinologist finally listened. She said: ‘Stick with one brand. Even if it’s generic. Don’t let the pharmacy switch you.’
That one rule saved my life.
So thank you for writing this. For people like me who were told we were ‘overreacting’ - you gave us language. You gave us proof.
And yes - I still side-eye any pill that doesn’t look exactly like the last one. And I’m not sorry.
Dee Monroe
January 26, 2026 AT 01:51There’s something deeply spiritual about medicine, isn’t there? We reduce it to molecules and percentages, but the human body doesn’t live in a spreadsheet. It lives in rhythm - in the quiet mornings, the unspoken fears, the weight of a pill in your palm.
When you take a drug, you’re not just ingesting chemistry. You’re trusting a system. You’re trusting the person who prescribed it. You’re trusting the factory worker who pressed the tablet. You’re trusting the pharmacist who didn’t switch your bottle without telling you.
Generics are a miracle of modern science - yes. But they’re also a mirror. They reflect how much we value care over cost, dignity over convenience, patience over profit.
Maybe the real question isn’t whether the AUC matches - but whether we, as a society, still believe that healing should be accessible, consistent, and human.
And if the answer is yes - then we must protect the system, not because it’s perfect - but because it’s the closest thing we have to justice in a pill.
Ben McKibbin
January 26, 2026 AT 19:16Let’s cut through the noise. The data is overwhelming: generics work. Period.
Yes, there are outliers. Yes, NTI drugs need extra care. But the moment you start treating every generic like it’s a lottery ticket, you’re not being cautious - you’re being reckless. You’re undermining the entire public health infrastructure that keeps millions alive.
And let’s be honest - the people who scream ‘it doesn’t work!’ are the same ones who won’t get bloodwork done, won’t tell their doctor they switched, and then blame the pill when their mood tanks.
It’s not the generic. It’s the silence.
Doctors don’t need more fear. They need more data. And patients don’t need more suspicion - they need more communication.
So if you’re worried? Talk to your doctor. Get tested. Don’t just assume the worst. That’s not health literacy. That’s fear masquerading as vigilance.
Generics aren’t the enemy. Complacency is.
Melanie Pearson
January 28, 2026 AT 16:07It is imperative to note that the regulatory framework governing bioequivalence standards is not only scientifically rigorous but also constitutionally aligned with the public interest in affordable therapeutics. The 80-125% confidence interval, as codified in the Hatch-Waxman Act of 1984, represents a statistically validated equilibrium between efficacy and economic accessibility.
Furthermore, the assertion that individual variability constitutes a systemic failure is a fallacy of composition. The FDA’s post-marketing surveillance system, which includes MedWatch and adverse event reporting, has demonstrated an efficacy rate exceeding 99.9% for approved generics.
It is therefore not merely advisable but ethically incumbent upon all stakeholders to refrain from propagating anecdotal narratives that undermine the integrity of evidence-based pharmacology. To do otherwise is to endanger public trust and to enable the proliferation of medical misinformation.
One must ask: if the science is this robust, why does emotional resonance override empirical data? The answer lies not in pharmacokinetics - but in psychology.
Uju Megafu
January 30, 2026 AT 06:18Oh my god. I knew it. I KNEW IT. This is all a lie. The FDA is just a puppet for Big Pharma. Did you know that Teva is owned by a company that also owns a factory in China that was caught dumping chemicals into rivers? And now they’re making my blood pressure pills? No wonder I feel dizzy.
And don’t get me started on the fillers. They put gluten in there. And MSG. And maybe even microchips. I read a blog post from a guy who said his neighbor’s cat got sick after eating a generic pill. Coincidence? I think not.
Also, why are all the generics white? White means ‘empty.’ They’re draining the power out of the medicine. It’s spiritual warfare.
My cousin in Nigeria told me her sister died because the generic had ‘invisible poison’ in it. She said the pills looked ‘too clean.’ That’s how they get you. They make it look safe so you don’t question it.
Wake up. This isn’t medicine. It’s control.
Barbara Mahone
January 31, 2026 AT 16:48I’ve been taking generic metformin for 12 years. Switched between six manufacturers. Never had an issue. My A1C is stable. My energy is fine.
My mom took generic lisinopril for 15 years. Never had a stroke. Never had a heart attack. She’s 87 and still gardens.
Generics aren’t magic. But they’re not magic tricks either.
They’re just medicine. Made by people. Tested by science. Regulated by a system that, yes, isn’t perfect - but has saved more lives than any conspiracy theory ever could.
Don’t fear the pill. Fear the silence between you and your doctor.
And if you’re worried? Ask for a blood test. Not a rant.
Kelly McRainey Moore
February 1, 2026 AT 22:13