Calcineurin Inhibitor Side Effect Calculator
Side Effect Risk Assessment
Select your medication and current dose to see the likelihood of common side effects.
When you’ve had a kidney, liver, or heart transplant, or are being treated for a severe autoimmune disease like lupus nephritis, calcineurin inhibitors like cyclosporine and tacrolimus can mean the difference between life and rejection. These drugs keep your immune system from attacking your new organ-but they come with a heavy price. Side effects aren’t rare. They’re common. And for many people, they shape daily life more than the transplant itself.
How Calcineurin Inhibitors Work
Cyclosporine and tacrolimus don’t just weaken your immune system-they target it precisely. They block calcineurin, a protein that turns on T-cells, the immune system’s frontline soldiers. No calcineurin? No interleukin-2. No T-cell activation. No organ rejection. Simple. Effective. But this precision comes with collateral damage.
These drugs were developed decades ago: cyclosporine in the 1970s, tacrolimus in the 1980s. Today, nearly 90% of kidney transplant patients in the U.S. take tacrolimus. Cyclosporine is now used in only about 10% of cases. Why? Because tacrolimus works better at preventing rejection. But better outcomes don’t mean fewer problems.
Nephrotoxicity: The Silent Threat
The biggest danger with both drugs? Your kidneys. Nephrotoxicity isn’t a side effect-it’s a defining feature of calcineurin inhibitors.
Acute nephrotoxicity hits fast. Within weeks of starting either drug, creatinine levels often rise 20-50%. That’s your kidneys struggling. The cause? Blood vessels in the kidneys constrict too tightly. It’s reversible if caught early. But if you stay on high doses too long, chronic damage sets in. Scar tissue builds up in the tubules. Grafts fail. Studies show chronic CNI toxicity causes nearly 40% of late kidney transplant losses.
Doctors now know: lower is better. The goal isn’t to suppress your immune system as hard as possible. It’s to find the smallest dose that still works. That’s why most transplant centers now monitor blood levels weekly when adjusting doses. For tacrolimus, the sweet spot is 5-10 ng/mL. For cyclosporine, it’s 100-200 ng/mL. Go above that? Risk spikes. Go below? Rejection risk rises.
Neurotoxicity: Tremors, Parkinsonism, and Brain Fog
Tacrolimus is far more neurotoxic than cyclosporine. Up to 70% of people on tacrolimus get tremors-shaky hands, difficulty holding a cup, writing, or buttoning a shirt. Cyclosporine? Only 10-25%.
It’s not just tremors. Some patients develop something far worse. A 2022 case report described a kidney transplant patient who developed full-blown parkinsonism-rigidity, slow movement, freezing-just two weeks after starting tacrolimus. When they switched to cyclosporine, symptoms vanished. Then, after eight months on cyclosporine, the tremors came back. That’s how powerful and unpredictable these drugs can be.
Even subtler effects matter. Around 15-20% of tacrolimus users develop mild cognitive changes: trouble focusing, memory lapses, brain fog. That’s why some transplant centers now do formal neurocognitive tests at three months. If your brain feels “off,” it’s not just stress. It could be the drug.
Diabetes: The Hidden Metabolic Trap
Tacrolimus doesn’t just hurt your kidneys and brain-it can wreck your pancreas. New-onset diabetes after transplant (NODAT) happens in 15-30% of tacrolimus users. For cyclosporine? Only 5-15%.
Why? Tacrolimus blocks the calcineurin-NFAT pathway in pancreatic beta cells. That’s the same pathway that tells your body to release insulin. No insulin? Blood sugar spikes. Many patients don’t know they’re diabetic until they’re dizzy, thirsty, or losing weight. By then, damage may already be done.
Doctors now act fast. If your fasting blood sugar climbs above 100 mg/dL, they don’t wait. They start SGLT2 inhibitors-drugs like dapagliflozin-that protect your heart and kidneys while lowering blood sugar. In one trial, this cut diabetes progression by 38%.

High Blood Pressure, Low Magnesium, High Potassium
Both drugs are brutal on your electrolytes. Over half of users develop hypertension. That’s not just a number-it means your heart and blood vessels are under constant strain.
Magnesium? Vanishes. Up to 60% of patients become hypomagnesemic. That causes muscle cramps, irregular heartbeats, and fatigue. Most need daily magnesium supplements just to stay stable.
Potassium? Rises. Hyperkalemia happens in 20-35% of users. High potassium can stop your heart. That’s why labs check potassium levels every time you refill your prescription.
Cyclosporine’s Cosmetic Toll
If you’re on cyclosporine, you might notice things no one talks about: hair where it shouldn’t be, gums that swell and bleed.
Hirsutism-excess facial or body hair-shows up in 20-30% of women. It’s not just annoying. It’s emotionally crushing. One patient told her transplant nurse, “I stopped going out because I looked like a man.”
Gingival hyperplasia? Your gums swell, bleed, and overgrow. Brushing feels impossible. Dentists often recommend gum surgery. This doesn’t happen with tacrolimus. That’s why many patients switch-despite the tremors and diabetes risk.
GI Issues: Nausea, Diarrhea, and Lost Appetite
Tacrolimus is harder on your stomach. Nausea hits 30-45% of users. Diarrhea? 25-40%. Cyclosporine? Only 15-25% and 10-20%, respectively.
Some patients lose 10-15 pounds in the first few months. They can’t eat. They’re tired. Their meds taste awful. One Reddit user wrote: “I take tacrolimus with peanut butter to mask the taste. If I skip it, I get rejected. If I take it, I feel like I’m dying.”
Quality of Life: The Real Cost
A 2022 study used a 100-point scale to measure how transplant patients feel. Those on CNIs scored 15-22 points lower than those off them. That’s like living with constant flu.
Survey data from 2,874 patients shows 78% would gladly switch to a drug with fewer side effects-if it worked just as well. That’s not just preference. It’s desperation.
On patient forums, tacrolimus users talk about sleepless nights from tremors. Cyclosporine users talk about hiding their hairy faces. Both groups say they feel like they’re surviving, not living.
What’s Changing Now?
Doctors aren’t ignoring this. The field is shifting. The goal isn’t just to keep your organ alive-it’s to keep you healthy.
Three big changes are happening:
- CNI minimization: For low-risk patients, doctors now reduce CNI doses by 50% after three months and add other drugs like mTOR inhibitors (sirolimus, everolimus).
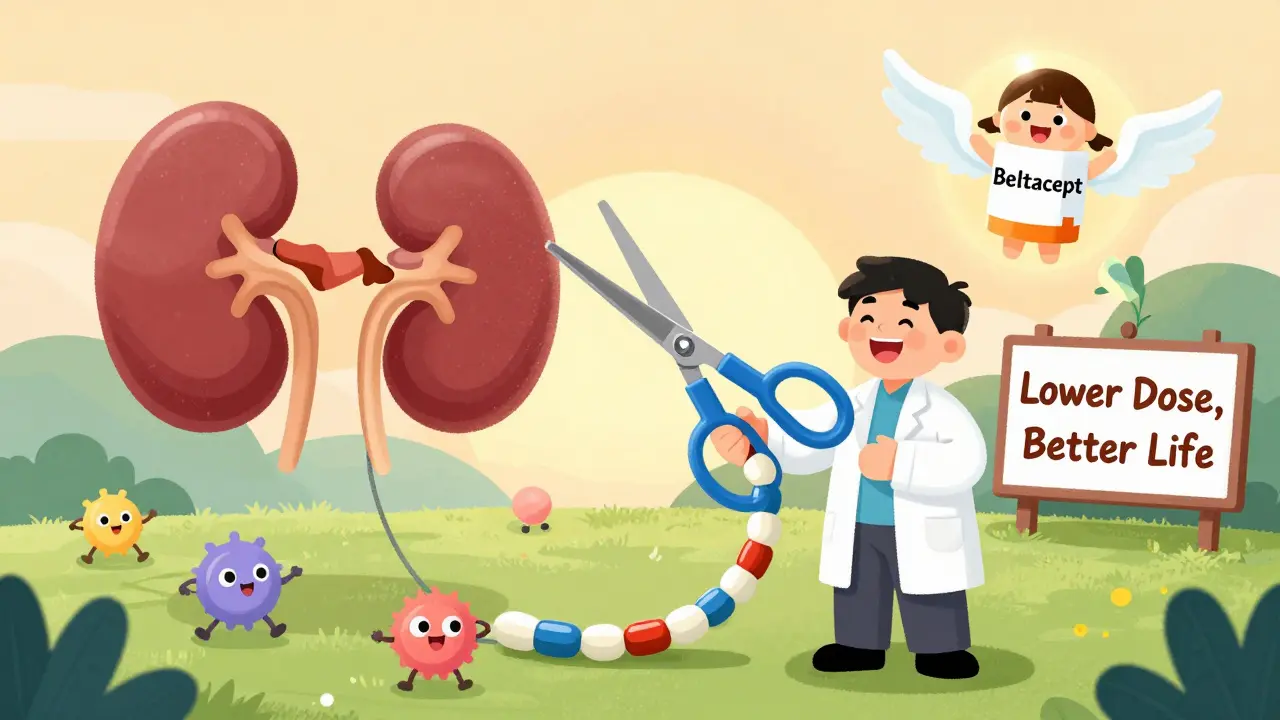
- CNI-free regimens: Belatacept, a newer drug, works without calcineurin inhibitors. In a 2023 trial, it matched transplant survival rates but gave patients 10 mL/min higher kidney function and far fewer metabolic problems.
- Early withdrawal: Some centers now stop CNIs entirely after six months for low-risk patients. Early results show 89% graft survival-with 40% fewer side effects.
Even new drugs are coming. Voclosporin, approved in 2021 for lupus nephritis, causes 30% less high blood pressure than cyclosporine. It’s not perfect-but it’s a step forward.
What Should You Do?
If you’re on cyclosporine or tacrolimus:
- Get your blood levels checked every week during dose changes, then monthly if stable.
- Check creatinine, potassium, magnesium, and fasting glucose every month.
- If you have tremors, ask: Can my dose be lowered? Is tacrolimus the cause?
- If your gums swell, see a dentist. Ask about switching to tacrolimus.
- If you’re gaining weight, peeing often, or feeling thirsty-get your blood sugar tested. Don’t wait.
- Ask your doctor: Am I a candidate for CNI minimization or withdrawal?
There’s no perfect drug. But there’s a better way to use these drugs. It’s not about taking more. It’s about taking less-and smarter.
Can you stop taking cyclosporine or tacrolimus after a transplant?
Yes, but only under strict medical supervision. For low-risk transplant patients, doctors may reduce the dose or switch to a CNI-free regimen after 6-12 months. Stopping abruptly can cause organ rejection. Always work with your transplant team before making any changes.
Which has worse side effects: cyclosporine or tacrolimus?
It depends on what you’re comparing. Tacrolimus causes more tremors, diabetes, nausea, and diarrhea. Cyclosporine causes more hair growth, swollen gums, and high blood pressure. Both harm the kidneys. Tacrolimus is more effective at preventing rejection, but its side effects are harder to live with for many patients.
Do calcineurin inhibitors cause weight gain?
Not directly. But they can lead to weight gain indirectly. High blood sugar from tacrolimus increases hunger and fat storage. Steroids (often taken with CNIs) cause fluid retention and appetite spikes. Some patients gain 10-20 pounds in the first year-not from the CNI alone, but from the full combo.
How often should blood levels be checked for cyclosporine and tacrolimus?
During dose adjustments, check weekly. Once stable, monthly is standard. Some centers test every two weeks for the first three months. Trough levels (just before your next dose) are the key measurement. For tacrolimus, aim for 5-10 ng/mL. For cyclosporine, 100-200 ng/mL.
Are there alternatives to cyclosporine and tacrolimus?
Yes. Belatacept is a CNI-free option approved for kidney transplants with better kidney function and fewer metabolic issues. Sirolimus and everolimus are mTOR inhibitors used in CNI-sparing regimens. Voclosporin is a newer CNI with less hypertension. But none are perfect. The choice depends on your risk of rejection, side effect tolerance, and kidney function.
Can calcineurin inhibitors cause long-term kidney damage?
Yes. Chronic CNI exposure causes scarring in the kidneys called interstitial fibrosis and tubular atrophy. This damage is often irreversible and accounts for up to 38% of late kidney transplant failures. That’s why modern protocols focus on using the lowest effective dose and switching to CNI-free regimens when possible.
Final Thoughts
These drugs saved your life. But they’re not harmless. They’re powerful tools-and like any tool, they need careful handling. The best outcomes don’t come from taking the most. They come from taking the least you can get away with.
If you’re struggling with side effects, you’re not alone. And you don’t have to live with them. Talk to your team. Ask about alternatives. Ask about lowering your dose. Your health after the transplant matters just as much as the transplant itself.

 Health and Wellness
Health and Wellness
Martin Viau
January 1, 2026 AT 06:48CNI minimization is just band-aid medicine. We're treating symptoms while ignoring the systemic failure of 1970s pharmacology. If we're still relying on calcineurin inhibitors in 2024, we're not advancing-we're institutionalizing toxicity. Belatacept isn't an alternative-it's the bare minimum we should've adopted a decade ago. The fact that 90% of patients are still on tacrolimus is a scandal, not a standard.
And don't get me started on the 'low-risk' patient myth. Who decides who's low-risk? A spreadsheet? A resident who hasn't seen a rejection in five years? We're gambling with lives using arbitrary thresholds while the drugs slowly fry the very organs we're trying to save.
Marilyn Ferrera
January 2, 2026 AT 09:32Every side effect listed here-nephrotoxicity, neurotoxicity, diabetes, gingival hyperplasia-is a direct consequence of inhibiting a fundamental cellular signaling pathway. Calcineurin isn't just a target; it's a guardian of homeostasis. We're not just suppressing rejection-we're dismantling the body's natural regulatory architecture. And yet, we call this 'medicine.'
Perhaps the real question isn't 'how to reduce side effects'-but 'why do we still accept this level of collateral damage as inevitable?'
Robb Rice
January 3, 2026 AT 13:34It's important to note that while the data on CNI toxicity is compelling, we must also acknowledge the overwhelming evidence supporting their efficacy in preventing acute rejection. The trade-off, while significant, has historically saved countless lives. That said, I completely agree with the push toward CNI-sparing regimens-especially in low-risk populations. I've seen patients on belatacept experience improved GFR and fewer metabolic complications, and it's encouraging. However, careful monitoring remains essential-any deviation from protocol can lead to catastrophic outcomes.
Also, please ensure your potassium levels are checked monthly, not just when you're symptomatic. Hypokalemia is silent until it's not.
-Robb, RN, Transplant Coordinator
Harriet Hollingsworth
January 4, 2026 AT 11:09So let me get this straight-we’re giving people drugs that turn their gums into overgrown seaweed, make their hands shake like they’re having a seizure, and turn their pancreas into a broken vending machine… and we call it a ‘win’?
What kind of sick, twisted logic is this? You don’t get to call someone ‘alive’ if they’re just barely breathing, covered in hair, diabetic, and too scared to leave the house because they look like a yeti.
This isn’t medicine. It’s torture with a prescription pad.
Someone needs to sue these drug companies. And the doctors who keep prescribing this garbage like it’s candy.
Deepika D
January 5, 2026 AT 12:33Hey everyone, I’m a nephrology nurse in Delhi, and I’ve seen this play out with over 200 transplant patients over the last 12 years. Let me tell you-this post is spot on, but here’s what no one talks about: the silence. Patients don’t complain because they’re terrified to sound ungrateful. ‘I got a new kidney, so I should be happy,’ they say. But then they cry in the waiting room because they can’t button their shirt or hold their grandchild.
I’ve had women stop going to weddings because their faces are hairy. I’ve had men quit jobs because they can’t type without shaking. I’ve had kids tell me their parents don’t hug them anymore because their gums bleed when they kiss.
It’s not just about labs. It’s about dignity. And yes, switching to belatacept or reducing CNIs isn’t just ‘an option’-it’s a moral imperative. If your doctor says ‘it’s fine,’ ask for a second opinion. Ask for a pharmacist consult. Ask for a quality-of-life score. You deserve more than survival. You deserve to live.
And if you’re reading this and you’re on tacrolimus? You’re not weak. You’re not ungrateful. You’re human. And your pain matters.
❤️ You’re not alone. Reach out. We’re here.
Bennett Ryynanen
January 7, 2026 AT 04:39Bro, I’ve been on tacrolimus for 8 years. Tremors? Yeah. Diabetes? Yep. Gums so swollen I can’t eat pizza anymore? Absolutely.
But here’s the thing-I’m alive. My kidney’s working. I’m not on dialysis. So yeah, I’m a mess. But I’m a mess with a second chance.
That said-I dropped my dose from 7mg to 4mg last year after my doc finally listened. My tremors cut in half. My sugar’s under control. My dentist says my gums are healing.
Don’t just accept it. Push. Ask for the lowest dose. Ask for belatacept. Ask for a switch. You’ve got a voice. Use it. Your life after the transplant? It’s yours to fight for.
And if your doc acts like you’re being dramatic? Find a new one. I did. Best decision I ever made.
Stay strong, fam. 💪
Chandreson Chandreas
January 7, 2026 AT 22:13Man, this hits different. I’m 3 years post-liver transplant, on cyclosporine. Hirsutism? Yeah, I got a beard now. My wife says it’s ‘charming.’ I say it’s a prison.
But here’s the thing-I switched from tacrolimus because of the brain fog. I couldn’t remember my kid’s birthday. That’s not a side effect. That’s a soul loss.
Cyclosporine’s got its own demons, but at least I can think. I take my meds with peanut butter like the guy said. Tastes like regret, but it’s my regret.
Don’t give up. Don’t shut down. Talk to your team. Find your dose. Find your balance. We’re all just trying to be human again.
🫂 You got this. And hey-your hair? It’s just hair. You’re still you.
-Chandreson, 3-year survivor
Darren Pearson
January 9, 2026 AT 02:58The assertion that calcineurin inhibitors are antiquated is both reductive and empirically unsound. While newer agents such as belatacept demonstrate promising outcomes in select cohorts, they are not universally applicable. The cost-benefit analysis of CNI-based regimens remains favorable in high-immunologic-risk patients, where rejection rates are demonstrably lower. Furthermore, the longitudinal data supporting CNI minimization is still evolving, and the long-term graft survival data for CNI-free protocols beyond five years remains insufficient to warrant widespread adoption.
Moreover, the emotional anecdotes presented, while poignant, do not constitute clinical evidence. Medicine is not governed by subjective distress but by population-level outcomes and risk stratification. To conflate quality-of-life metrics with therapeutic efficacy is to misunderstand the foundational principles of transplant medicine.
That said, personalized medicine is the future-but it must be grounded in evidence, not emotion.